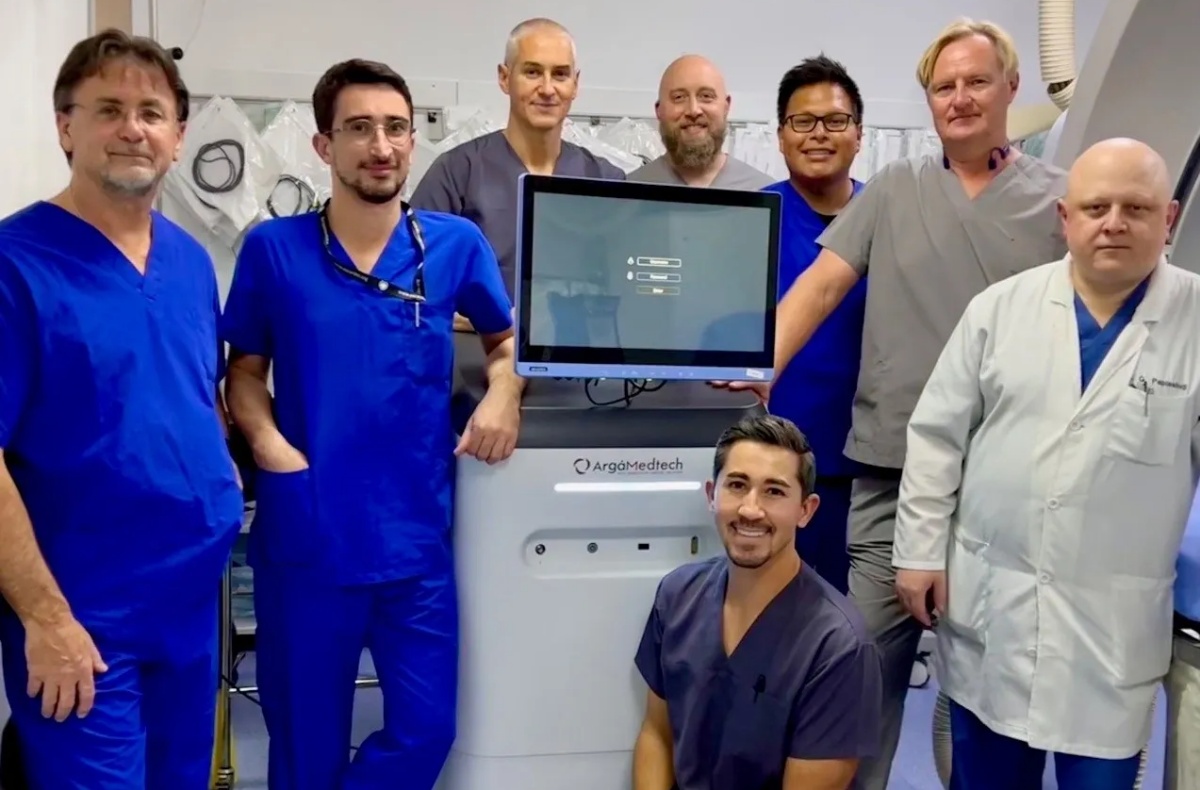
Swiss-US start-up Argá Medtech developing a next-generation cardiac ablation system for treating cardiac arrhythmias, including atrial fibrillation (AF), has closed a €54M oversubscribed Series B funding. The new funding will advance US and EU clinical studies and expand its US offices in San Diego.
Argá Medtech’s CSE ablation system enables electrophysiologists to treat any region in the heart safely and efficiently using a single, multi-configurable catheter while titrating lesion depths according to the location within the heart. The new funds will enable Argá Medtech to advance the development of its innovative CSE Pulsed Field Ablation (PFA) system for treating AF through the execution of an IDE study in the US and a CE Mark study in the EU. The company is based at Biopôle (Lausanne) and San Diego. With the funding, Argá will also expand its US offices in San Diego in anticipation of its US clinical activities.
The round was led by the existing investors, Advent Life Sciences (UK) and Earlybird Health (Germany), as well as new investor Gilde Healthcare (the Netherlands) and an undisclosed strategic investor.
“We are pleased to secure the support of such marquee investors who believe that Argá Medtech will revolutionize the atrial fibrillation ablation field,” said David Neale, CEO of Argá Medtech who is working out of Lausanne. “This financing enables us to advance toward our goal of validating the CSE PFA system in Europe and the US as we work to deliver a safe, fast, and effective treatment to millions of people affected by cardiac rhythm disorders and atrial fibrillation. We are proud of our accomplishments to date, including conducting a 48-patient first-in-human study in Europe, which demonstrated the high performance of our platform in treating atrial fibrillation.”
The company’s CSE PFA platform combines its proprietary CSE PFAgenerator with a multi-configurable catheter to provide unmatched flexibility in AF treatment. Argá Medtech’s CSE system uses a sinusoidal/sine wave, while other PFA platforms are typically powered by square wave energy sources. This approach offers several advantages, such as allowing physicians to configure the energy delivery and titrate the depth to the location in the heart. Additionally, the waveform is delivered through a versatile, all-in-one catheter that can be shaped to create circular, linear, or focal ablation lesions, eliminating the need to perform catheter exchanges to achieve the desired lesion set. Thus, the procedure is simplified, reducing the risk of introducing air bubbles as one catheter is removed and another is reinserted, as well as reducing costs.
AF is the most common heart arrhythmia, affecting about 38 million patients worldwide, rendering them five times more likely to have a stroke. While ablation using thermal energy has proven effective in alleviating AF symptoms, higher risks are associated with its use. In contrast, PFA energy delivery offers a tissue-selective, precise ablation of the intended heart tissue while preserving nearby tissues and minimizing the risks of thermal injury, such as those to the esophagus or the phrenic nerve.(Press release / ES)






















































Please login or sign up to comment.
Commenting guidelines